Read the most important and the latest news about CERVANTES trial here
I. CERVANTES study (protocol version v2.0) is now under evaluation in Clinical Trial Information System (CTIS) for authorisation in Austria, Belgium, Italy, Poland and Spain.
Austria - 5 sites
Belgium - 5 sites - authorised
Italy - 1 site
Poland - 5 sites
Spain - 6 sites
II. CERVANTES Portuguese translation under preparation for Brazilian International Review Board (IRB) - registration of 3 sites
III. Let' get together; ESGO 2026 CERVANTES meeting. Come and find out more about the possibility to open CERVANTES at your site and what news we have.
Friday 27.2.2026 18:00-18:30 ESGO meeting room 1


Current status


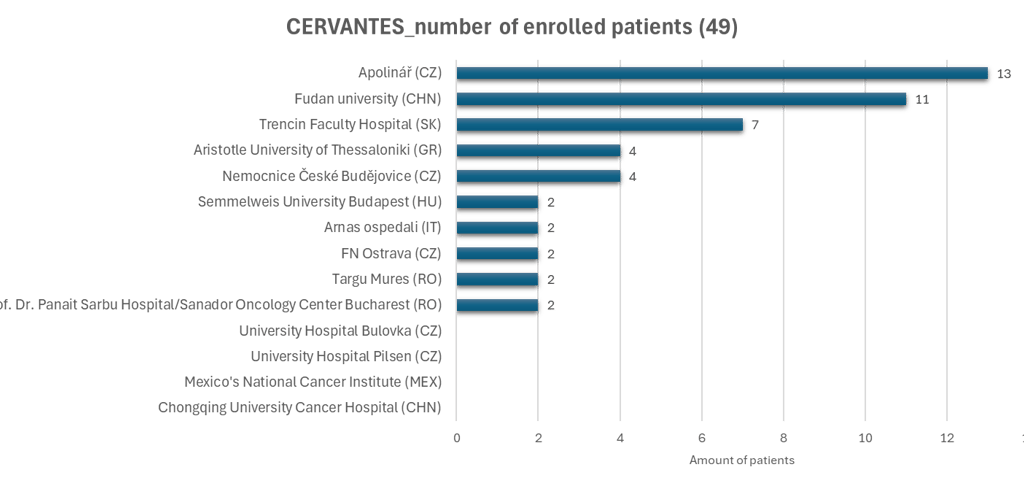
Status updated 19/01/2026
Subscribe for CERVANTES Trial
newsletter*
